Short background
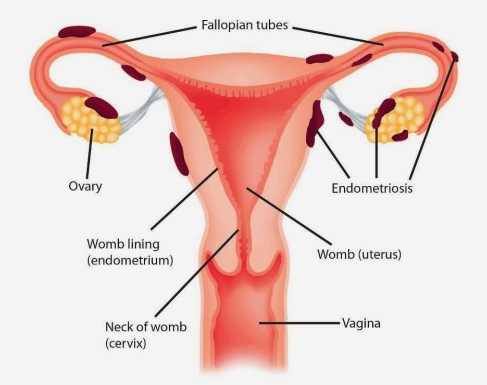
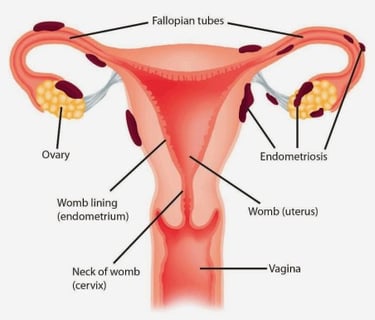
According to the World Health Organization, at least 10% of fertile women suffer from endometriosis. However, women during their teenage and after menopause also may have clinical signs of the disease. In about 30% endometriosis affects the patients' fertility.
Unfortunately, we do not know the exact reason for endometriosis development. Many scientific theories are trying to explain the endometriosis etiology. They include a specific immune response to the natural process of menstruation, the dissemination of uterine mucous cells via blood or lymphatic vessels, congenital presence and further activation of the cells resembling the uterine mucous tissue, and many other theories.
Clinical signs
Endometriosis may manifest in different complaints including:
pelvic and abdominal pain during menstruation which may irradiate the back, groin, and legs.
painful ovulation
painful intercourse including superficial and deep penetration.
irregular vaginal spotting and bleeding.
intestinal and bowel signs including abdominal swelling, diarrhea, constipation, incomplete emptying, and bloody stool.
urinary signs including painful, frequent, and urgent urination.
chest pain and blood caught in case of diaphragmatic endometriosis.
cyclic pain and bloody discharge on the abdominal wall area especially if the patient had the previous surgery (cesarean section, laparoscopy, or another open surgery) with the scar on the abdominal wall.
infertility as a consequence of adhesions in the area of uterine tubes and the potential effect on the oocyte quality.
As you may see there are numerous, diverse, and even weird signs that can be caused by endometriosis. Although the main characteristic of endometriosis is the worsening of the complaints during menstruation or ovulation, the women usually suffer from chronic pelvic pain which affects their daily activity and life quality.
It is important to note that although endometriosis may involve many organs with different severity of signs and symptoms, it is not a cancer. The absolute risk of malignant transformation is shallow and mainly related to the big atypically looking endometriod ovarian cysts.
Types of endometriosis
According to the localization, endometriosis is classified as gynecological, gastrointestinal (mostly bowel), urinary tract, and distally localized which may involve the diaphragm, lungs, abdominal wall, and other organs.
Endometriosis which affects only a superficial layer of the abdominal or pelvic cavity is called peritoneal endometriosis. When endometriosis affects deeper tissues we usually call it deep infiltrating endometriosis (DIE). The endometriosis which affects the ovaries contributing to the cyst formation is called endometrioma. But here is a trick, there is no correlation between the the grade of disease and the severity of the symptoms. The patient with peritoneal endometriosis can suffer not less than the patient with DIE, and vice versa.
Diagnosis
Another trick is difficulty in the endometriosis diagnosis. After we ask specific questions, we perform an abdominal and gynecological examination and ultrasound examination. We can refer the patient to the targeted ultrasound or MRI tests when it is needed. However, even if all the tests did not reveal any specific sign, the endometriosis can be highly suspected if the complaints are very suggestive.
Conservative treatment
Endometriosis is a chronic, autoimmune, and hormone-dependent condition. The treatment is based on those characteristics and includes hormonal treatment, pain medications, nutritional changes, physiotherapy, and alternative options like acupuncture.
Since endometriosis is an estrogen-dependent condition we use a long-term hormonal treatment to suppress an endogenous estrogen release from the ovaries. This suppression is temporary and can be terminated when the patient desires to get pregnant.
Surgical treatment
In cases when the conservative treatment is not helpful or when the patient cannot be hormonally treated (medical contraindications, hyper-sensitivity to the hormones, pregnancy desire) the specialist may consider surgical treatment. The surgery is mainly performed using the minimally invasive (laparoscopic) approach with 5-10 mm incisions on the abdominal wall. The surgery may include the adhesions separation, superficial and/or deep endometriosis resection, ovarian cyst removal, the biopsy of the tissue suspected of endometriosis, and other modalities.
Two main points before the surgery:
Whatever stage and type of endometriosis the patient has, it is paramount to consult with a specialist in the endometriosis field who has a holistic approach to the disease and its consequences!
Whatever surgical technique is chosen for women who desire to get pregnant, the first priority is the fertility potential preservation!
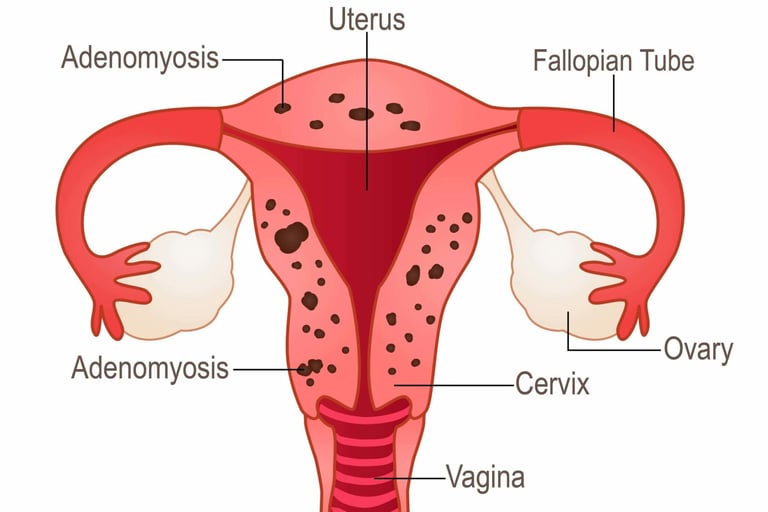
The word "adenomyosis" means the presence of glands in the muscular layer of the uterus. From this definition, we understand that this condition is characterized by the growth of the uterine lining glands into a deeper muscular layer of the uterine wall.
This condition is another type of endometriosis that is present in the uterus itself. It causes the uterus to thicken and enlarge in some cases up to double or triple its usual size. Adenomyosis can cause painful periods, heavy or prolonged menstrual bleeding with clotting, and abdominal/pelvic pain.
This condition also contributes to the infertility development. During pregnancy adenomyosis increases the risk of miscarriage and premature labor.
Adenomyosis can be diagnosed by gynecological examination, ultrasound, and MRI. We distinguish two main types of adenomyosis:
focal adenomyosis, which occurs in one particular site of the uterus.
diffuse adenomyosis, which spreads throughout the muscular uterine layer.
As for endometriosis due to the estrogen sensitivity of adenomyosis, this condition is managed with long-term hormonal treatment and pain medications. Adenomyosis symptoms often go away after menopause. However, if the conservative treatment is not helpful and the patient has no birth plan, the specialist may consider a surgical treatment that includes a hysterectomy (uterus removal) which represents a definitive treatment of the adenomyosis.
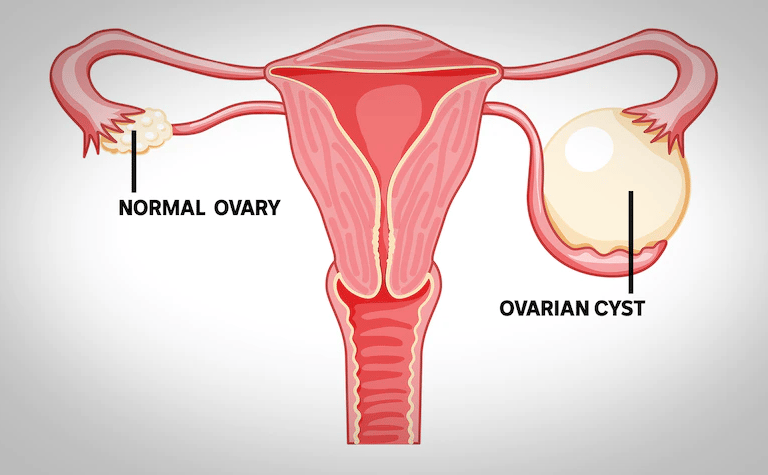
Ovaries are relatively small organs in the pelvis that hold egg cells and release hormones, such as estrogen and progesterone.
An ovarian cyst is a sac filled with fluid or semisolid material that forms on or within one or both of the ovaries. There are different types of ovarian cysts, most of which are painless and harmless (benign). Usually, ovarian cysts don’t cause symptoms. The patient likely won’t know she has one unless the specialist finds one during a routine pelvic exam or imaging procedure. Rarely, ovarian cysts can cause complications.
Functional cysts
They represent the most common type of ovarian cyst and aren’t disease-related.
Follicular cysts. A small sac in the ovary, called a follicle, releases an egg each month as part of the menstrual cycle. A follicular cyst forms when the follicle doesn’t release an egg. Instead, the follicle fills with fluid and grows bigger.
Corpus luteum cysts. After the follicle releases an egg, it forms a hormone-producing group of cells called the corpus luteum. A cyst forms when fluid collects in the corpus luteum, causing it to grow.
Functional cysts generally shrink over time, usually within 60 days, without specific treatment.
Abnormal cysts
Cystadenomas. These cysts form on the surface of the ovary. They can be filled with thin and watery fluid or thicker and mucous-like.
Dermoid cysts (mature teratomas). Dermoid cysts consist of cells that make up all types of tissue in the human body, ranging from skin, hair, teeth, and even brain tissue.
Endometriomas. These cysts are filled with old blood and inflammatory factors.
Ovarian cancer. Unlike the conditions above, ovarian cancer cysts (tumors) are solid masses of cancer cells.
Signs and symptoms
Pelvic pain or a dull ache in your back. Extreme pain, nausea, and vomiting may be the signs of ovarian torsion which prevents blood flow to the ovary.
A feeling of fullness (bloating) located in the lower belly may feel more pronounced on one side of the body.
Pain during intercourse (dyspareunia).
Painful periods.
Hormone-related problems include obesity, increased growth of body hair, and infertility.
Diagnosis
We usually use the following tests to diagnose an ovarian cyst:
Pelvic exam
Ultrasound can detect cysts on the ovaries, including their location and whether they are primarily fluid or solid.
Computer tomography (CT) is usually done if the malignancy is suspected to determine the spread of the tumor.
Magnetic resonance imaging (MRI) is used for endometriomas and endometriosis-related lesions.
Blood tests that can indicate the risk of a non-benign finding (tumor markers such as CA 125).
Laparoscopy is a minimally invasive surgical procedure performed under general anesthesia. We perform laparoscopy in the selected cases of persistent or abnormal cysts. The advantage of laparoscopy is that we can treat the cyst at this time.
Treatment
As we say functional ovarian cysts usually go away without treatment. However, in cases of persistent or complex cysts, laparoscopic cyst removal can be considered. During this procedure, we insert a small camera through a small incision in the abdomen. We rigorously revise all the organs of the abdominal and pelvic cavity including reproductive organs. The ovarian cyst can be removed through tiny incisions (ovarian cystectomy).
One of the main limitations of the surgical treatment of ovarian cysts is possible damage to the healthy ovarian tissue that may cause a decrease in fertility. To avoid this the surgeon must combine deep knowledge in the field of reproduction and have high surgical skills using different surgical techniques for maximal ovarian tissue preservation.
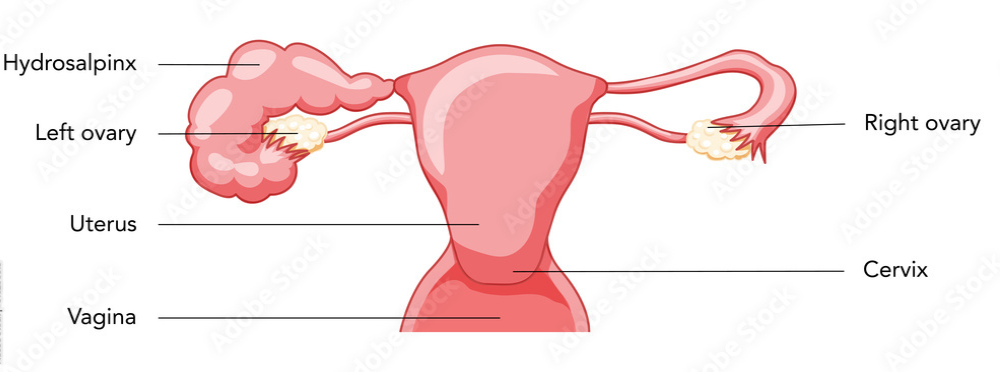
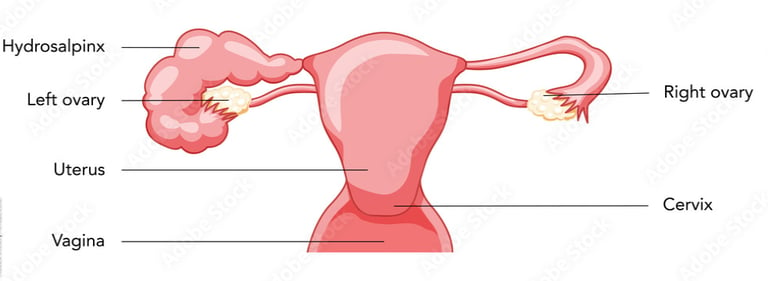
The term "hydrosalpinx" comes from Greek, with hydro meaning water and salpinx meaning tube.
Hydrosalpinx is the blockage of a woman’s fallopian tube caused by a fluid buildup and dilation of the tube at its end. Most often it occurs at the fimbrial end of the tube next to the ovary, but it can also occur at the other end of the tube that attaches to the uterus.
Blocked fallopian tubes are one form of tubal factor infertility. When the fallopian tube is blocked, the cells inside the tube secret fluid that can’t escape, dilating the tube. This prevents fertilization – and thus pregnancy – by blocking an ovulated egg from moving from the ovary to the fallopian tube for fertilization by the sperm. If an ovulated egg is somehow able to connect with a sperm for fertilization, the hydrosalpinx would still likely block the resulting embryo from traveling to the uterus for implantation and pregnancy. It can also potentially cause an ectopic pregnancy, in which the embryo implants outside the uterus, most often inside the fallopian tube, and results in a life-threatening situation.
Hydrosalpinx may be caused by:
pelvic infections
previous pelvic surgery
endometriosis
some tumors
What tests can be used to diagnose hydrosalpinx?
Ultrasound. Normal fallopian tubes aren’t usually visible on an ultrasound. But if they’re swollen because of fluid build-up, they’ll appear larger than usual.
Hysterosalpingogram (HSG). An HSG is an X-ray dye test to check for blockages in the fallopian tubes. It’s the most common test used to diagnose hydrosalpinx. If the dye spills out of your tubes and into your pelvic cavity, your tubes are open. If the dye stops, your tubes are blocked.
Laparoscopy is a minimally invasive surgery that allows to perform a tube test (chromotubation). Laparoscopy can be used to confirm the results of an HSG and to treat hydrosalpinx.
Hydrosalpinx can also negatively impact fertility treatment. According to the National Institutes of Health, when hydrosalpinx fluid is present in a woman undergoing assisted reproductive technologies such as IVF, it reduces the success of such treatments by half compared with woman who do not have hydrosalpinx. For this reason, women wanting to conceive via IVF are often counseled to have the hydrosalpinx surgically removed before IVF treatment.
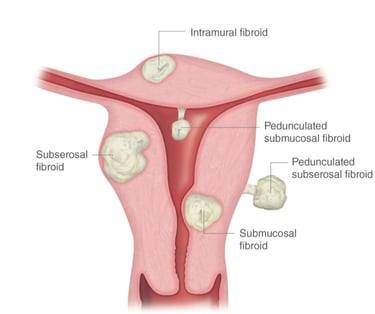
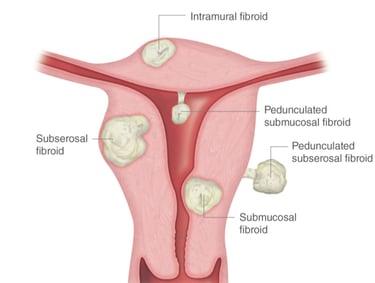
Uterine myomas (fibroids) are a common type of benign tumor of uterine muscle. About 80% of all women have uterine fibroids. The exact cause is unknown, but healthcare providers believe the hormones estrogen and progesterone play a role.
Not all fibroids cause symptoms, but when they do, symptoms can include:
increased abdominal distention (enlargement)
heavy menstrual bleeding,
back pain,
frequent urination,
constipation
pain during intercourse (dyspareunia).
There are different types of uterine fibroids depending on where they’re located and how they attach. The location of the fibroid usually explains the clinical signs it causes. Specific types of uterine fibroids include:
Intramural fibroids: These fibroids are embedded into the muscular wall of your uterus. They’re the most common type.
Submucosal fibroids: These fibroids grow under the inner lining of your uterus. This type of fibroids is usually responsible for heavy menstrual bleeding or bleeding between the menstruations causing anemia.
Subserosal fibroids: This type of fibroid grows under the lining of the outer surface of your uterus. They can become quite large and grow into your pelvis and cause back pain, frequent urination, and constipation due to their pressure on the adjusted organs.
Pedunculated fibroids: The least common type, these fibroids attach to your uterus with a stalk or stem. They’re often described as mushroom-like because they have a stalk and then a wider top.
Fibroids can grow as a single nodule (one growth) or in a cluster. Clusters of fibroids can range in size from 1 millimeter to more than 20 centimeters (8 inches) in diameter or even larger.
The uterine myomas can be diagnosed using:
Pelvic exam
Ultrasonography
Magnetic resonance imaging (MRI)
Computed tomography (CT) scan
Hysterosalpingography(HSG)
Sonohysterography is when the provider places a small catheter in your vagina and then injects saline into your uterus. This extra fluid helps to create a clearer image of your uterus than you would see during a standard ultrasound.
Small fibroids often don’t need treatment, but larger fibroids can be treated with medications or surgery.
Medications that we use to shrink the fibroid include:
Hormonal medications including birth control pills and gonadotropin-releasing hormone analogs contribute to the fibroid shrinkage due to hormonal suppression.
Pain medications: These medications help manage pain and discomfort caused by fibroids. OTC medications include acetaminophen and ibuprofen.
Iron supplements: If you have anemia from excess bleeding, your provider may also suggest you take an iron supplement.
The surgical treatment is considered in cases of significant symptomatic fibroids when the conservative treatment has failed. The type of procedure that may work best will depend on where the fibroids are located, and how big they are. The types of myomectomy (fibroid removal) procedures can include:
Hysteroscopy is when we insert a small camera into the uterus through the vagina. Then we use small manipulators to cut away and remove the fibroids.
Laparoscopy. In this procedure, we use a few small incisions in the abdomen to remove the fibroids. We use a special device (morcellator) to cut and remove the fibroids from the abdominal belly to avoid the widening of incisions.
Laparotomy. In extreme cases when the fibroids are huge and very symptomatic we make one larger incision in your abdomen and remove the fibroids through this one cut.
If the woman does not plan future pregnancy, there are additional surgical options that may be considered according to the size and location of fibroids:
Hysterectomy (uterus removal) is a definitive treatment of fibroids. By removing the uterus completely, the fibroids can’t come back and your symptoms should go away. If your ovaries are left in place, you won’t go into menopause after a hysterectomy, but will not have the menstrual bleeding. This procedure might be recommended if you’re experiencing very heavy bleeding from your fibroids or if you have large fibroids. Minimally invasive hysterectomies include laparoscopic or robotic methods.
Uterine fibroid embolization is when an interventional radiologist puts a small catheter in your uterine artery and injects small particles, which then block the flow of blood from the artery to the fibroids. Loss of blood flow shrinks the fibroids and improves the symptoms.
Since uterine fibroid treatment may include a few modalities and approaches, the deep knowledge of the reproduction field and extensive surgical skills of the surgeon are critically essential.
Uterine (endometrial) polyps are growths in the inner lining of the uterus (endometrium).
The polyp attaches to the endometrium by a thin stalk or a broad base and extends into the uterine cavity. Polyps may range in size from a few millimeters to a few centimeters. There may be one or several polyps present. Uterine polyps are usually benign (noncancerous), but they may cause problems with periods (irregular menstruations) or infertility.
Uterine polyps are sometimes asymptomatic, meaning they do not cause symptoms. For this reason, many people with uterine polyps may never receive a diagnosis. Research does suggest that polyps are more common in certain populations. For instance, they are more common in people who have gone through menopause than those who have not.
The symptoms of uterine polyps include:
Irregular menstrual periods (unpredictable timing and flow).
Heavy flow during menstrual periods.
Bleeding or spotting between periods (intermenstrual bleeding).
Infertility.
Vaginal spotting or bleeding after menopause (red, pink, or brown blood).
Bleeding after intercourse.
These tests for diagnosis of uterine polyp include:
Ultrasound exam.
Sonohysterography is when we send a sterile fluid into the uterus through a thin tube called a catheter. The fluid causes the uterus to expand, providing a clearer image of any growth within the uterine cavity during the ultrasound procedure.
Hysteroscopy, is when we insert a thin tube with a lighted telescope (hysteroscope) through the vagina and cervix into the uterus. The hysteroscope allows us to examine the inside of the uterus. Hysteroscopy is sometimes used in combination with surgery to remove uterine polyps.
Endometrial biopsy uses a soft plastic catheter to collect tissue from the uterine cavity. The sample is tested in a laboratory to detect any abnormal cells.
Curretage using an instrument with a small loop on the end that's used to scrape tissue or polyps. The removed tissue or polyps may be sent to the laboratory for testing to determine if cancer cells are present.
Treatment will depend on the symptoms and other factors that increase the risk of uterine cancer. If the patient is still in the reproductive years and the polyp isn’t causing symptoms, we may consider monitoring the polyp instead of treating it. The polyp may go away on its own. If the patient has gone through menopause and/or if polyps are causing symptoms, we may need treatment.
Methods of treatment include:
Medications that keep the hormones balanced, like progestins or gonadotropin-releasing hormone agonists, may be used to relieve symptoms.
Uterine polypectomy is when we remove the polyp during hysteroscopy. A hysteroscope allows us to insert tools that can be used to excise (cut) and remove polyps.
Hysterectomy is a surgery that involves removing the uterus and may be necessary in cases where the polyps contain cancer cells. This surgery is usually performed by a minimally invasive (laparoscopic) approach without large scars like during cesarean section.
Normal menstrual flow typically lasts about five days and occurs every 21 to 35 days. Abnormal uterine bleeding (formerly called menometrorrhagia) is when the bleeding happens between monthly periods or when periods are extremely heavy and/or prolonged.
Menorrhagia is excessive and/or prolonged menstruation.
Metrorrhagia is excessive, prolonged, and/or irregular bleeding unrelated to menstruation.
Abnormal uterine bleeding can have many causes, including a variety of medical conditions and even stress.
Hormone imbalances
Anovulation
Pituitary gland dysfunction
Polycystic ovary syndrome(PCOS).
Structural abnormalities in your uterus
Polyps
Fibroids
Adenomyosis
Precancer and cancer
Uterine cancer
Cervical cancer
Vaginal cancer
Ovarian cancer
Endometrial hyperplasia
Infections
Trichomoniasis.
Cervicitis
Chlamydia
Gonorrhea
Endometritis.
Vaginitis
Other medical conditions
Von Willebrand disease
Liver disease
Kidney disease
Pelvic inflammatory disease
Leukemia or platelet disorders.
Retained foreign bodies and trauma
Medications
Blood thinners and aspirin.
Hormone replacement therapy.
Tamoxifen(breast cancer drug).
Intrauterine devices(IUDs).
Birth control pills and injectables
We may order several tests or procedures when diagnosing abnormal uterine bleeding. These tests may include:
A pregnancy test.
Blood clotting and complete blood count.
Hormone levels test.
Hysteroscopy to check for fibroids, polyps, or signs of cancer.
Pelvic ultrasound to check for any growths in reproductive organs that may be causing the bleeding.
Endometrium biopsy to collect tissue samples from the uterus lining and check for signs of cancer or pre-cancer cells.
Medications and surgical options are available to manage the bleeding or treat what’s causing it.
Medications
Birth control pills
Progestin (can be given by a shot, implant, or device placed in the uterus called an IUD).
Gonadotropin-releasing hormone (GnRH) agonists and antagonists can temporarily stop or reduce bleeding by preventing ovulation and bleeding related to fibroids (uterine myomas).
Surgery
Hysteroscopy. A procedure to remove atypical structures in the uterus, like fibroids and polyps.
Uterine artery embolization. Stops blood flow to fibroids, causing them to shrink.
Myomectomy. Removes fibroids while keeping the uterus intact and preserving your ability to get pregnant and have children.
Endometrial ablation. Destroys the uterus lining through the use of a laser, heat, electricity, microwave energy, or freezing. The patient shouldn't have this procedure in case of pregnancy desire.
Hysterectomy. Removes the uterus and represents a definitive treatment of abnormal uterine bleeding.
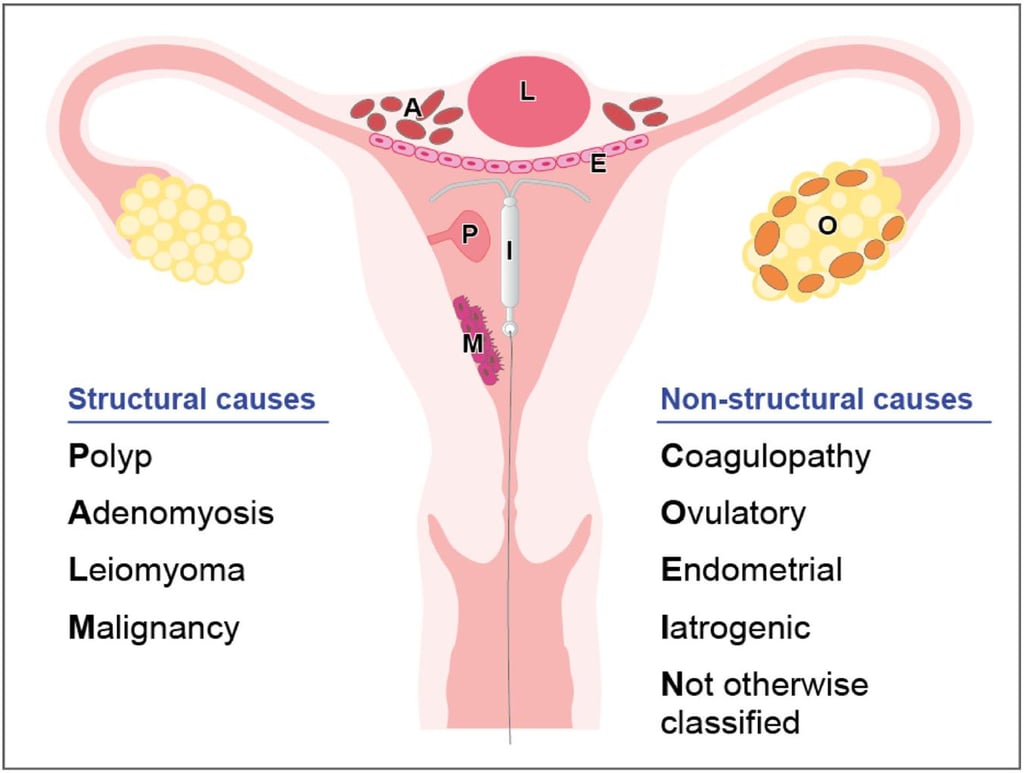
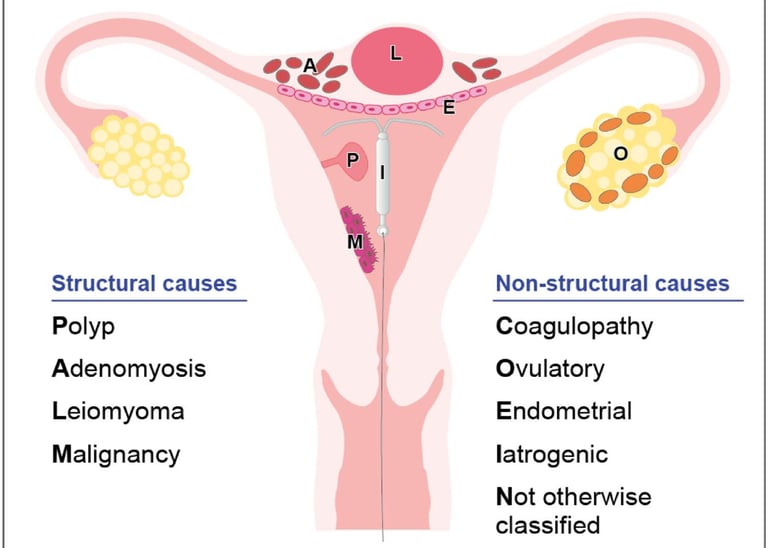
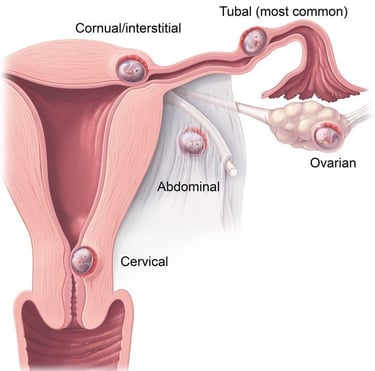
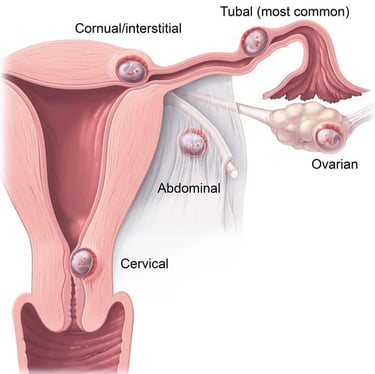
An ectopic (extrauterine) pregnancy is a pregnancy that happens outside of the uterine cavity. This occurs when a fertilized egg implants in a location that can’t support its growth. An ectopic pregnancy most often happens in the fallopian tube which connects the ovaries and uterus. Ectopic pregnancies more rarely can occur on the ovary, abdominal cavity, or cervix. Pregnancies can’t continue if they’re ectopic because only the uterus is meant to carry a pregnancy. Ectopic pregnancies occur in about 2% of all pregnancies.
Ectopic pregnancies can become life-threatening, especially if your fallopian tube breaks (ruptures). This is a ruptured ectopic pregnancy, and it can cause severe abdominal and vaginal bleeding. Unfortunately, an ectopic pregnancy is fatal for the fetus and your pregnancy can’t continue. This is a medical emergency.
Several risk factors could increase the chance of developing an ectopic pregnancy:
A previous ectopic pregnancy.
A history of pelvic inflammatory disease (PID), an infection (including certain sexually transmitted diseases) that can cause scar tissue to form in the fallopian tubes, uterus, ovaries, or cervix.
A history of surgery on the fallopian tubes (including tubal ligation) or the other pelvic organs.
A history of infertility.
Endometriosis
An Intrauterine device (IUD) relatively increases the risk of EUP.
A history of smoking tobacco.
Early symptoms of an ectopic pregnancy can be very similar to typical pregnancy symptoms. However, the patient may experience additional symptoms during a developing ectopic pregnancy, including:
Vaginal bleeding.
Pain in the lower abdomen, pelvis, and lower back.
Dizziness or weakness.
If your fallopian tube ruptures, the pain and bleeding could be severe enough to cause additional symptoms. These can include:
Fainting.
Low blood pressure.
Shoulder pain.
Rectal pressure or bowel problems.
To diagnose ectopic pregnancy we perform blood tests and ultrasound to determine the pregnancy location.
We treat ectopic pregnancies with medication or surgery:
in most cases, close monitoring is enough, because most ectopic pregnancies will terminate spontaneously.
In some cases, we may suggest using a medication called Methotrexate to stop the fertilized egg from growing, ending the pregnancy.
In rare cases of active abdominal bleeding or when the ectopic pregnancy does not respond to Methotrexate we perform a laparoscopic surgery. We may consider removing the uterine tube with the ectopic pregnancy (salpingectomy) if the fallopian tube has ruptured or if it is at risk of rupture.
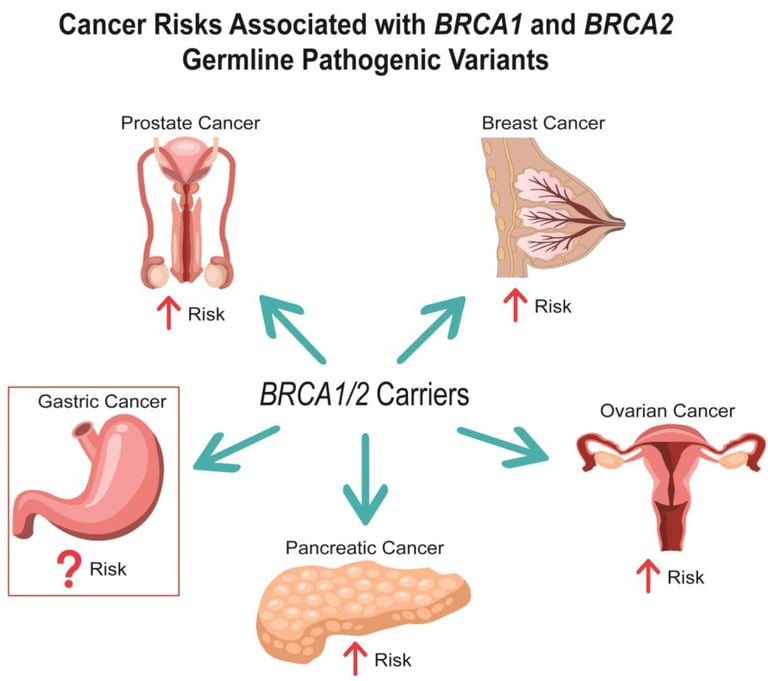
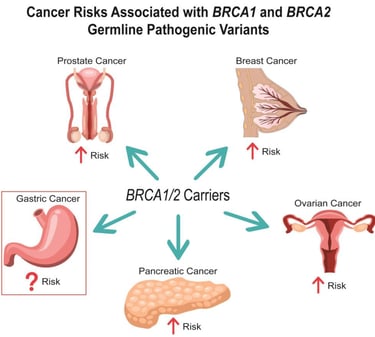
BRCA testing identifies changes in BRCA1 or BRCA2 gene. BRCA genes produce proteins that are involved in DNA repair. Abnormal changes (mutations) in BRCA genes increase the risk of breast cancer, as well as ovarian and other cancers.
BRCA mutations are inherited meaning, they are passed down from parents and run in families. BRCA testing requires a single vial of blood.
We recommend BRCA testing if a patient has:
A personal history of breast cancer, especially cancer diagnosed before age 50.
A relative has already been diagnosed with a BRCA mutation.
One or more family members who’ve had breast cancer, especially male breast cancer.
Received a diagnosis of both breast and ovarian cancer.
Ashkenazi Jewish ancestry.
A personal or family history of multiple cancer diagnoses.
A family history of an inherited cancer predisposition disorder.
In addition to breast cancer BRCA mutations can also increase the risk of:
Ovarian cancer and two related cancers: fallopian tube cancer and peritoneal (abdominal lining) cancer.
Prostate cancer in males.
Pancreatic cancer.
Fanconi anemia (rare cancer that develops during childhood).
Testing positive for a BRCA mutation significantly increases the risk of cancer. However, it doesn’t mean you’ll definitely get cancer. Possible next steps include:
More frequent cancer screenings (such as mammograms).
Additional screening techniques (such as breast MRI).
Limited use of birth control pills may reduce the risk of ovarian cancer but can raise the risk of breast cancer in some people.
Preventive mastectomy (healthy breast tissue removal) before cancer can develop.
Preventive removal of ovaries and fallopian tubes to reduce the risk of developing ovarian cancer. This surgery is usually performed by laparoscopic approach without a large abdominal scar like during a cesarean section.
If prophylactic oophorectomy (ovary removal) is recommended for a woman who plans a pregnancy in the future, fertility preservation should be considered, including egg/embryo freezing before the surgery. After the surgery at the appropriate time, we prepare the patient's endometrium using hormonal treatment, thaw the eggs/embryos, and return them to the uterus.
Infertility is a condition of the reproductive system that causes people to be unable to get pregnant.
Getting pregnant involves several steps:
The brain must produce reproductive hormones that control ovarian function.
An egg must mature in the ovary.
The ovary must release an egg (ovulation).
The fallopian tube must pick up the egg.
Sperm must travel up the vagina and through the uterus to the fallopian tube.
The sperm should fertilize the egg to create an embryo.
The embryo should successfully travel through the fallopian tube to the uterus where it implants.
Types of infertility include:
Primary infertility when the patient has never been pregnant and can’t conceive after one year (or six months after the age of 35r) of regular, unprotected sexual intercourse.
Secondary infertility when the patient cannot get pregnant again after having at least one pregnancy.
There are many causes of infertility, and sometimes, there isn’t a simple answer as to why the woman can not get pregnant. Some causes of infertility affect just one partner, while others affect both partners. Risk factors for infertility include:
Age, particularly being in the late 30s or 40s for women. For men, age begins affecting fertility closer to 50.
Eating disorders, including anorexia and bulimia.
Excessive alcohol consumption.
Exposure to environmental toxins, such as chemicals, lead, and pesticides.
Over-exercising.
Past radiation or chemotherapy.
Sexually transmitted infections(STIs).
Smoking and using tobacco products. This behavior plays a role in about 13% to 15% of infertility cases.
Substance abuse.
Having obesity or being underweight.
Abnormalities of the hormone-producing centers of the brain (hypothalamus or pituitary gland).
Some chronic conditions and diseases.
Twenty-five percent of infertile couples have more than one factor that contributes to their infertility.
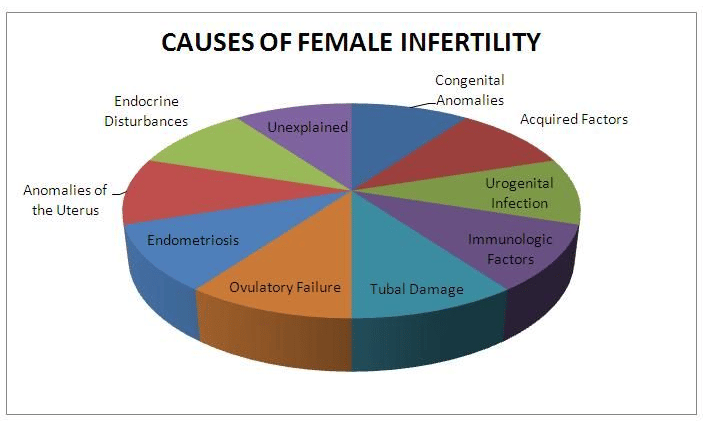
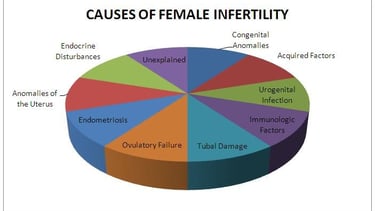
These factors can contribute to female infertility:
Endometriosis
Structural abnormalities of the vagina, uterus, or fallopian tubes.
Autoimmune conditions like celiac disease or lupus.
Kidney disease
Pelvic inflammatory disease(PID).
Hypothalamic and pituitary gland disorders.
Polycystic ovary syndrome(PCOS).
Primary ovarian insufficiency or poor egg quality.
Sickle cell anemia.
Uterine myoma (fibroid) or uterine polyp.
Thyroid disease.
Prior surgical sterilization (tubal ligation or removal).
Genetic or chromosomal disorders.
Sexual dysfunction.
Surgical or congenital absence of the ovaries.
Infrequent or absent menstrual periods.
Numerous factors contribute to problems with the shape, movement (motility), or amount (low count) of sperm. These causes of male infertility include:
Enlarged veins (varicocele) in the scrotum, the sac that holds the testicles.
Genetic disorders, such as cystic fibrosis.
Chromosomal disorders, such as Klinefelter syndrome.
High heat exposure to the testicles from tight clothing, and frequent use of hot tubs and saunas.
Injury to the scrotum or testicles.
Low testosterone (hypogonadism).
Misuse of anabolic steroids.
Sexual dysfunction, such as erectile dysfunction, anejaculation, premature or retrograde ejaculation.
Undescendent testicles.
Previous chemotherapy or radiation therapy.
Surgical or congenital absence of testes.
Prior surgical sterilization (vasectomy).
The cause of infertility is determined based on the results of tests that check three main groups of problems:
Ovulation factor
Mechanical factor (cervix, uterine cavity, uterine tubes)
Male factor
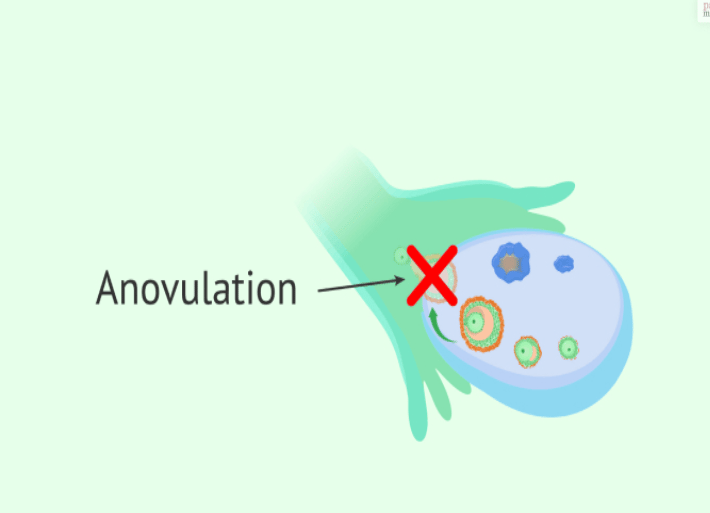
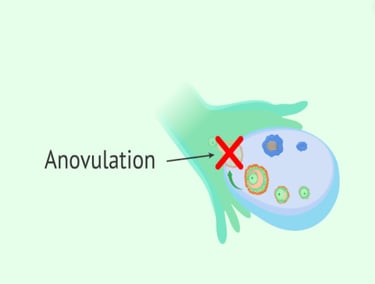
Ovulation is the release of an egg (ovum) from the ovary. On average, it happens on day 14 of a 28-day menstrual cycle. There are multiple hormones involved in ovulation. Anovulation happens when an egg is not released from the ovary during the menstrual cycle. Anovulation is a common condition and is the cause of approximately 25% of infertility cases.
If any of the following conditions or situations apply, the patient is more likely to experience anovulation:
Having just started getting periods
When the body transitions to menopause (perimenopause).
Decreased ovarian reserve.
Polycystic ovary syndrome (PCOS).
Adrenal gland issues.
Pituitary gland disorders, such as Cushing syndrome or Acromegaly.
Damage to or illness of the kidneys, liver, and/or thyroid.
Certain medications, such as anabolic steroids.
Very low body mass index (BMI), which is usually either from having anorexia or from doing long-term excessive exercise.
Breastfeeding.
Experiencing excessive stress.
Signs and symptoms of anovulation can include:
Irregular periods.
Very heavy or light periods.
A lack of periods (amenorrhea).
Tests that can help diagnose anovulation include:
Blood progesterone levels.
Blood thyroid levels.
Blood prolactin levels
Other pituitary hormone levels including follicle stimulation hormone (FSH) and luteinizing hormone (LH).
Ultrasound exam of the pelvic organs.
Treatment for anovulation depends on correcting the hormonal imbalance that’s causing it:
Stress managing.
Weight managing.
Decreasing exercise frequency and intensity.
Treat specific chronic medical conditions.
Adjusting current medications.
Oral medications for ovarian stimulation (Letrozole, Clomiphene citrate).
Human chorionic gonadotropin (hCG) injection causing the ovary to release an egg.
Follicle-stimulating hormone (FSH) injections for controlled ovarian stimulation.
Gonadotropin-releasing hormone (GnRH) agonists and antagonists medications to control the levels of luteinizing hormone (LH) the body releases, which is needed for ovulation.
Controlled ovarian hyperstimulation as a first step of In vitro fertilization (IVF) treatment.
The prognosis for anovulation depends on the cause of the anovulation. In most cases, anovulation can be treated with lifestyle changes, fertility medications, or medications that treat the condition that’s causing the anovulation.
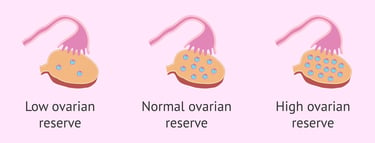
Diminished ovarian reserve is when the patient has fewer eggs (oocytes) in the ovaries compared to people of the same age. Diminished ovarian reserve makes it harder to get pregnant.
Actual numbers of eggs vary from person to person, but these are the average number of eggs that a woman has throughout her life:
Birth: 1 to 2 million eggs.
Puberty: 300,000 to 400,000 eggs.
Age 40: 25,000 eggs.
Menopause: less than 1,000 eggs.
Aging is one of the biggest causes of low ovarian reserve. However, sometimes, there is no clear cause. Some genetic disorders and previous medical treatments cause diminished ovarian reserve. These include:
Genetic disorders that affect the X chromosome.
Past radiation or chemotherapy(cancer treatment).
Past surgery on the ovaries.
Losing one or both of the ovaries.
Autoimmune conditions.
A woman can still get pregnant with diminished ovarian reserve.
Ovarian reserve testing involves:
Blood tests to measure levels of anti-Mullerian hormone (AMH) or follicle-stimulating hormone (FSH) and estradiol. These tests measure the potential response to ovarian stimulation or fertility medications.
Vaginal ultrasound to measure ovarian volume and count the number of follicles in the ovaries. This is called antral follicle count (AFC).
Unfortunately, there is no way to reverse diminished ovarian reserve or produce more eggs. If the patient is at risk for low ovarian reserve, we may suggest freezing the eggs or embryos before the egg count declines further. Egg freezing involves taking hormones to stimulate the ovaries to make as many eggs as possible. Then, the mature eggs are collected and frozen for future In vitro fertilization (IVF) treatment.
If the egg quality and/or quantity are poor, we may suggest using donor eggs. In this case, the partner's sperm fertilizes an egg from a donor. The resulting embryo is then transferred to the uterus.

Some males are more likely than others to experience infertility. The risk factors for male infertility include:
Overweight or obesity.
Age 40 or older.
Radiation exposure.
Environmental toxins exposure including lead, calcium, pesticides, or mercury.
Tobacco, marijuana, or alcohol use.
Medications include cyproterone, flutamide, spironolactone, bicalutamide, cimetidine, or ketoconazole.
Exposure to heat that raises the temperature of the testes. Those who frequently use a sauna, hot tub, or wheelchair might experience this.
A history of undescended testicle(s).
A history of varicoceles, which are widened veins in the scrotum.
Exposure to medications containing testosterone.
Many biological and environmental factors can impact sperm parameters. Possibilities include:
Azoospermia. The medical term is used when there is no sperm in the ejaculate. It can be “obstructive,” where there is a blockage preventing sperm from entering the ejaculate, or it can be “nonobstructive” when it is due to decreased sperm production by the testis.
Oligospermia. The production of low or poor-quality sperm.
Genetic diseases. Examples include Klinefeflter’s syndrome, myotonic dystrophy, microdeletion, and more.
Malformed sperm. Sperm that cannot live long enough to fertilize the egg.
Some medical conditions. Examples include diabetes, some autoimmune disorders, cystic fibrosis, and some infections.
Some medications and supplements.
Varicocele. This is a condition where the veins on the testicles are larger than normal, causing them to overheat, which can affect the shape or number of the sperm.
Cancer treatments. Chemotherapy, radiation, or surgery that removes the testicles (one or both).
Unhealthy habits. Substance use, including alcohol, smoking, and drugs.
Trauma to the testes.
Hormonal disorders that affect the hypothalamus or pituitary glands.
When male infertility is suspected, the first test we perform is semen analysis. It determines the following:
Sperm volume: amount of sperm per ejaculate.
pH: a measurement of acidity or basicity.
Sperm concentration: number of sperm per millimeter of semen.
Total sperm count: number of sperm in the whole ejaculate.
Motility: the sperm’s ability to move to the egg and fertilize it.
Velocity: how fast the sperm travels.
Linearity: how straight the sperm moves.
Morphology: size and shape of the sperm.
Color.
Viscosity: how fast the semen liquefies.
Viability, or ability to survive.
Leukocytospermia quantitation/Endtz test.
Other possible tests include:
Special staining for the azoospermic specimen.
Semen biochemistry fructose test.
Sperm antibody tests (direct and indirect immunobead).
Reactive oxygen species.
Sperm DNA assessment.
With modern technology and methods, the number of treatment options for male infertility has expanded. Depending on the cause of infertility, treatments may include:
Medications:
Hormone therapy to increase the number of sperm.
Lifestyle changes:
Maintain a normal body weight.
Stop smoking.
Stop drinking.
Stop using marijuana.
Stop any recreational drug use.
Surgeries:
Varicocelectomy. This is a surgical procedure that treats a varicocele. It reduces testicular pain and can increase male fertility.
Sperm Retrieval. In some severe cases, a biopsy of the testicle is required to find sperm.
In vitro fertilization (IVF). For some couples dealing with male infertility, IVF is the treatment of choice. During the IVF process, the ovaries are stimulated with injectable fertility medications to cause multiple eggs to mature. When the eggs are ready, they are collected in a minor procedure. Fertilization is accomplished by exposing the eggs to sperm in a culture dish, or by directly injecting a single sperm into each mature egg, a process called intracytoplasmic sperm injection (ICSI). After fertilization, embryo development is monitored over the next three to five days, and embryos are then placed into the uterus by way of a small catheter inserted through the cervix.
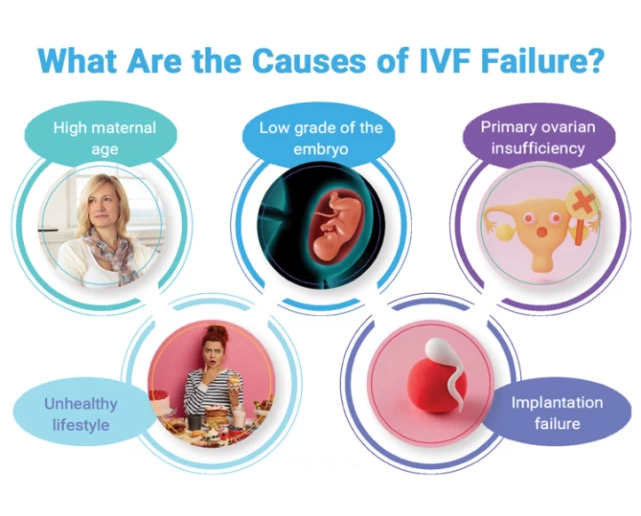
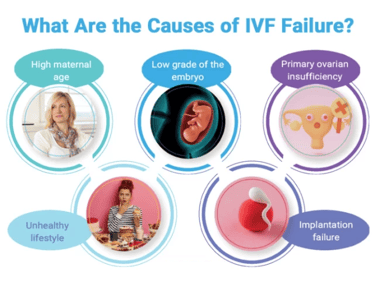
Recurrent implantation failure (RIF) refers to cases in which women have had three failed embryo transfers with good-quality embryos. The definition should also take advanced maternal age and embryo stage into consideration.
The failure of embryo implantation can be a consequence of uterine, male, or embryo factors, or the specific type of IVF protocol.
There are multiple risk factors for recurrent implantation failure including:
advanced maternal age.
smoking status of both parents.
elevated body mass index.
stress levels.
The tests to diagnose the reason for RIF may include:
Genetic consultation and analysis.
Evaluation of blood clotting.
Hysteroscopy to diagnose the uterine pathology.
Ultrasonography to exclude hydrosalpinx (the blockage and fluid accumulation in the fallopian tube) and other pelvic pathology.
Assessment for adenomyosis and endometriosis.
Assessment of Ovarian function including ovarian reserve tests.
Hormonal tests.
Evaluation of uterine lining receptivity (endometrium microarray test).
Evaluation of bacterial environment in the uterine cavity (endometrial cavity microbiome).
Sperm DNA fragmentation test.
Intracytoplasmic morphologically selected sperm injection (IMSI) requires examination of spermatozoa under ultra-magnification.
Therapeutic interventions for recurrent implantation failure include:
Lifestyle modifications (smoking and alcohol cessation, stress management, body weight optimization).
Optimization of IVF treatment protocol.
Preimplantation genetic screening or diagnosis to choose the optimal embryos for transfer.
Consideration of prescription of antithrombotic agents.
Immunotherapy.
Antibiotics for infection.
Therapeutic endometrial biopsy.
Hysteroscopy to correct uterine pathology (Polyps, myomas, adhesions, and septum).
Laparoscopic surgery to treat hydrosalpinx and/or endometriosis.